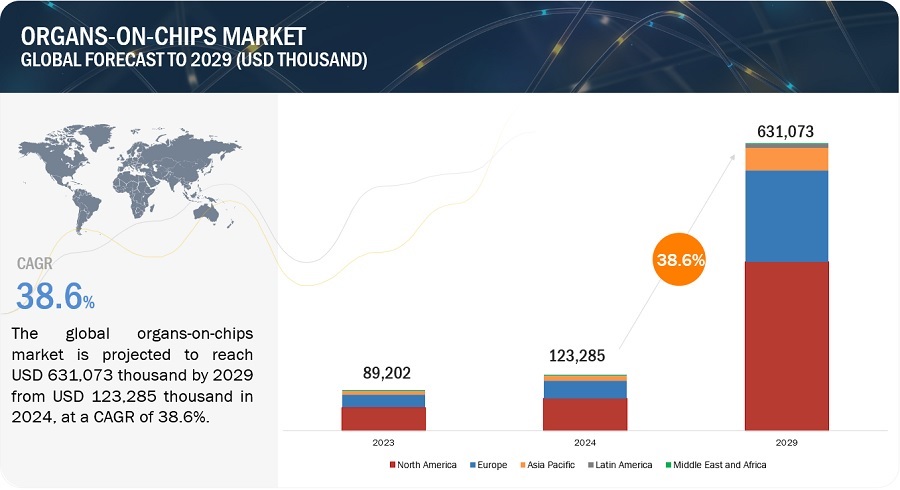
The size of global organ-on-chip market in terms of revenue was estimated to be worth $23,285 thousand in 2024 and is poised to reach $631,073 thousand by 2029, growing at a CAGR of 38.6% from 2024 to 2029. The comprehensive research encompasses an exhaustive examination of industry trends, meticulous pricing analysis, patent scrutiny, insights derived from conferences and webinars, identification of key stakeholders, and a nuanced understanding of market purchasing dynamics.
Organ-on-chip Market Dynamics
Driver: Increasing demand for animal free testing and growing government and private institution funding
Organ-on-chip technology is being developed as a potential substitute to animal drug testing. Often drugs in the final stages of pre clinical trials fail due to differences in animal and human physiology and drug metabolism. This high attrition of potential drug candidates and ethical concerns regarding use of animal for drug testing has lead to development of organ-on-chip technology reduce reliance on animals and to improve drug toxicity testing efficacy. Use of animals for testing of cosmetic drugs is banned in Europe since 2013. In 2023, the US FDA passed Modernization Act 2.0 to reduce animal drug testing and rely on alternative drug testing methods such as organ-on-chips.
Restraint: Application of organ-on-chip technology limited to pre clinical trials
Transitioning Organ-on-Chip (OOC) technology to the clinical phase of drug development faces several restrictions such as a limited access to renewable cell sources impacting model reliability, ethical concerns regarding specific cell types like induced pluripotent stem cells (iPSCs), and challenges in accurately representing the complexity of human organs during biological scaling. The low throughput of current OOC technology affect the scalability and efficiency of the model, impeding its integration into clinical research. To address these limitations, the implementation of parallelization and automation in OOC systems, known as High Throughput OOC (HT-OOC) technology, offers a promising solution for advancing drug development beyond preclinical stages, facilitating comprehensive testing of large compound libraries to identify relevant drugs for disease pathogenesis.
Opportunity: Development of multi-organ-on-chip systems
Muti-organ-on-chip systems are developed the functions of multiple organs and inter-organ communications enabling study of drug responses and evaluate drug toxicity for various organs simultaneously. Multi-organ chips (MOCs) are used for safety assessments in the cosmetic and food industries, providing a animal free and efficient compound testing methods. In cosmetics, miniaturized versions of human skin, eyes, and relevant organs, allow for safety evaluations of ingredients and finished products without animal testing. MOCs can be developed with individual specific cells allowing companies to assess interaction of specific compounds with unique skin biology. MOCs incorporating digestive organs allow evaluation of nutrients to develop personalized and nutritious food products. These chips also offer a alternative approach to toxicity testing, replacing traditional animal models.
Challenge: Lack of standardization and low throughput of current organ-on-chip technology
The Organ-on-Chip (OOC) technology is a relatively new and complex technology and therefore faces challenges primarily due to a lack of standardization and low throughput. Standardization is crucial for ensuring consistency and reliability in OOC systems, promoting comparability across different platforms. However, the absence of agreed-upon regulations for reporting experiment findings slow down progress. Additionally, limited scalability and low throughput are also major challenges, restricting large-scale screening studies and increasing costs, making accessibility a concern for smaller research groups and startups. Efforts are underway to address these challenges by developing advanced OOC technology, focusing on designing chips with multiple compartments and integrating automated handling systems.
The organ-based model accounted for the largest share of the model type segment in overall organ-on-chip industry in 2023.
On the basis of model type, the organ-on-chip market is segmented organ-based model and disease-based model. In 2023, the organ-based model segment accounted for the largest share of the market owing to a high drug development cost and limited efficacy of animal models for drug toxicity studies. Growing interest of pharma and biotechnology companies towards a animal free drug testing approach also supports the growth of this segment.
The liver-on-chip segment dominates the organ type segment in overall organ-on-chip industry in 2023.
On the basis of organ type, the organ-on-chip market is segmented into liver, kidney, lung, heart, intestine and other organs. Liver-on-chip accounted for the largest share of the market owing to factors such as high attrition of potential drug candidates due to human-hepatocytic drug toxicity which is not detectable in animal and 2D cell cultures. Liver-on-chip models allow mimicking of human liver functions and provide more efficient drug delivery and development opportunities.
North America was the largest market for overall organ-on-chip industry in 2023 and also during the forecast period.
Geographically, the organ-on-chip market is segmented into North America, Europe, Asia Pacific, Latin America, Middle East and Africa. The market was dominated by North America in 2023 and this dominance is anticipated to continue throughout the forecast period between 2023 and 2029. The market for organ-on-chip is expanding in the North America region as a result of factors like availability of government and private sector funding and developed organ-on-chip infrastructure.
The prominent players in the organ-on-chip market are Emulate, Inc. (US), SynVivo, Inc. (US), Nortis, Inc. (US), MIMETAS B.V. (Netherlands), TissUse GmBH (Germany), Netri (France), Insphero (Switzerland), Axosim (US), Obatala Sciences (US) among others.
Recent Developments of Organ-on-Chip Industry:
- In September 2023, CN Bio Innovations Ltd (UK) and LifeNet Health LifeSciences (US) partnered to provide validated human cells for CN Bio's Organ-on-a-Chip systems.
- In June 2023 TissUse GmbH (Germany) and PMI (US) entered a collaborative agreement to leverage PMI's InHALES technology along with TissUse's Multi-Organ-Chip (MOC) platform.
Contact:
Mr. Rohan Salgarkar
MarketsandMarkets Inc.
1615 South Congress Ave.
Suite 103, Delray Beach, FL 33445
USA : 1-888-600-6441
UK +44-800-368-9399
Email: [email protected]
Visit Our Website: https://www.marketsandmarkets.com/
Content Source: